The grant expands the Company’s focus on women’s health following our recent publication on the importance of CDK8/19 inhibition as treatment for breast cancer
CDK8/19 inhibition in peritoneal metastases suggests a potential new path.”
COLUMBIA, SC, UNITED STATES, January 6, 2025 /EINPresswire.com/ -- Senex Biotechnology announced today the award of a $400,000 Phase I Small Business Technology Transfer (STTR) grant from the National Cancer Institute, part of the National Institutes of Health (NIH). The grant will fund the development of a novel formulation of SNX631-6, the Company’s CDK8/19 Mediator kinase inhibitor and clinical candidate as a treatment for metastatic ovarian cancer (OC). Metastatic OC is the second most common gynecological malignancy and a lethal disease. Since metastatic OC is localized to the peritoneal cavity in most patients, this cancer is the primary target disease for intraperitoneal (IP) chemotherapy. Unfortunately, even IP therapy with the standard chemotherapeutic drugs eventually fails in peritoneal OC, and new targeted drugs against the metastatic disease are urgently needed. — Mythreye Karthikeyan, Ph.D., Co-PI, UAB Department of Medicine.
“Rigorous prior research by Senex’s collaborators has documented the role of CDK8/19 in peritoneal OC and provides a strong foundation for the success of an improved IP therapeutic approach to this difficult disease. There is strong commercial potential for the proposed delivery strategy for the anticancer drug, which could address a major need for better therapy of OC. This grant expands the Company’s focus on women’s health following our recent breakthrough publication describing the importance of CDK8/19 inhibition as a potential treatment for breast cancer (https://www.pnas.org/doi/10.1073/pnas.2414501121).” Dennis Goldberg, Ph.D., CEO, Senex Biotechnology.
“The devastating impact of peritoneal metastases, particularly from Gynecological cancers represents one of the most challenging unmet needs in oncology. Having lost my mother to this disease, I've seen firsthand how limited our current treatment options are. The promising preclinical data for CDK8/19 inhibition in peritoneal metastases suggests a potential new path forward for these patients who urgently need more effective therapies.” Mythreye Karthikeyan, Ph.D., Co-PI, Associate Professor, UAB Department of Medicine.
"Metastatic tumors are especially difficult to treat due to the exceptional plasticity of metastatic cells that allow them to adapt to different therapies. CDK8/19 inhibitors, which suppress such plasticity, are unique in being more effective against metastatic than primary tumors. This includes their efficacy against peritoneal OC, a lethal disease and a major unmet medical need.” Igor B. Roninson, Ph.D., Founder and Chief Science Officer at Senex Biotechnology.
This project will be supported by an NIH grant R41CA298223. The content of this press release is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
About Senex Biotechnology
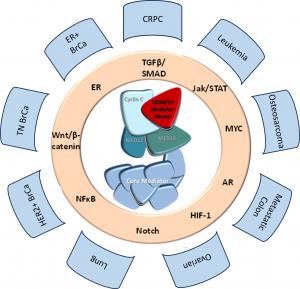
Senex Biotechnology is a drug discovery and development company focused on cancer therapeutics. Senex’s lead program targets CDK8/19, a protein that regulates gene expression and is required by cancer cells to adapt to adversarial conditions; such adaptation leads to cancer drug resistance and metastasis. Senex is developing highly selective small-molecule inhibitors of CDK8/19 for the treatment of presently incurable types of prostate cancer, breast cancer, osteosarcoma, ovarian cancer and leukemia. We are also investigating the utility of these inhibitors for different cancers in combination with other therapeutics, as well as for inflammation, cardiovascular and other diseases. SNX631-6, our novel, highly potent and selective drug candidate is anticipated to enter clinical trials in 2026.
Senex has recently obtained patents for the composition of matter of SNX631-6 and related compounds and their use for cancer treatment in the US and Europe. Related patent applications are in review in other countries around the world. Senex has a broad patent portfolio, including patents for the use of any CDK8/19 inhibitor for the treatment of breast and prostate cancer, and patent applications for targeted CDk8/19 degraders (PROTACs [Proteolysis Targeting Chimeras]).
Senex was founded by Dr. Igor Roninson, based on the discovery in his academic laboratory of a novel biological pathway associated with aging (senescence) and involved in cancer and other chronic diseases, as well as the use of functional genomics technologies to identify novel drug targets that are required by tumor cells but not by normal tissues. Senex has won 16 competitive grant awards from the National Institutes of Health, Department of Defense Congressionally Directed Medical Research Programs (DoD CDMRP) and the Alzheimer’s Drug Discovery Foundation. The Company’s work on breast cancer drug development was supported by grants from the National Cancer Institute.
For further information, visit www.senexbio.com.
Contact information:
Dennis I Goldberg
Senex Biotechnology, Inc.
+1 508-878-7589
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.