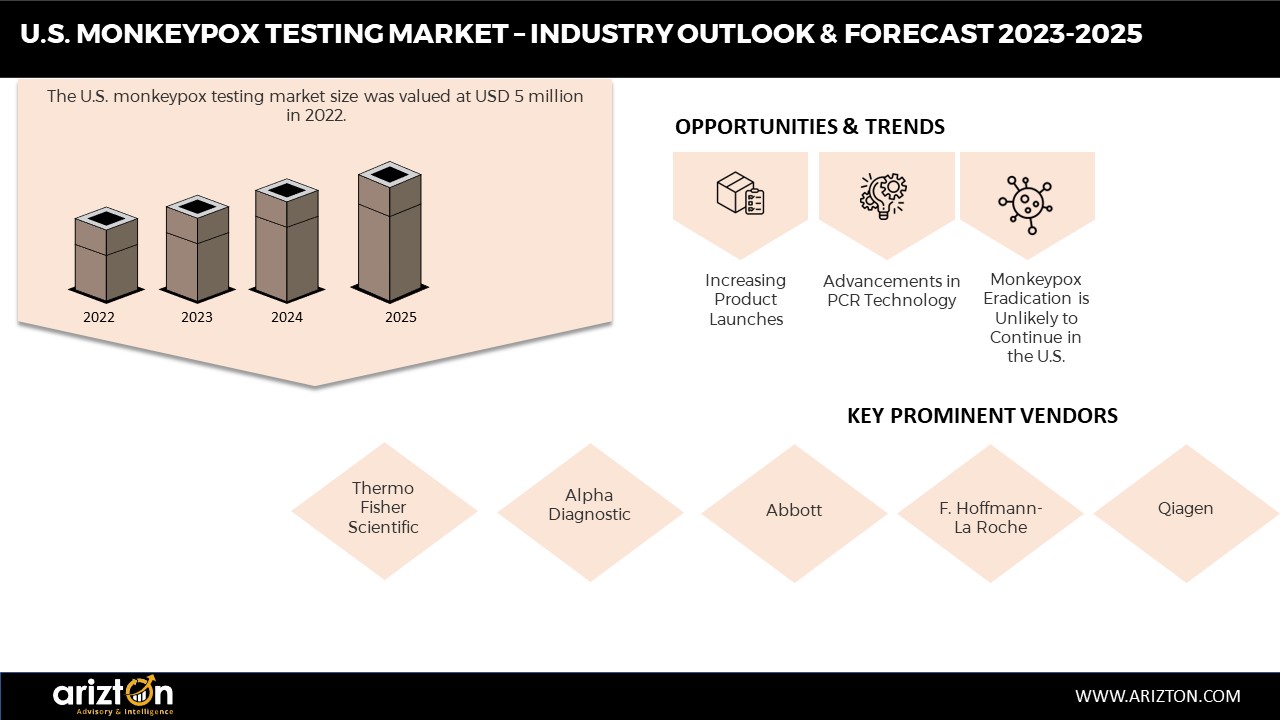
The U.S. monkeypox testing market size was valued at USD 5,336.29 thousand in 2022 and is expected to reach USD 91 thousand by 2025.
Request for free sample report: https://www.arizton.com/request-sample/3729
The U.S. Monkeypox Testing Market Size, Share, & Trends Analysis Report By
- Type: PCR, Lateral Flow Assay, and Others
- End-User: Laboratories, Hospitals & Clinics, and Others
- Geography: S. (Southern, Western, Northeast, and Central)
Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2023–2025
The COVID-19 pandemic made the U.S. healthcare system increase its testing capacity and handle outbreaks and pandemics better. The monkeypox virus was diagnosed through the PCR-based method, like the COVID-19 infection testing, and has helped the government immediately increase the testing and provide treatment to the infected people and contribute to the U.S. monkeypox testing market. From day one of this outbreak, providers had access to a high-quality, FDA-cleared test to detect monkeypox. The CDC has scaled the testing capacity to 78 sites in 48 states, primarily at state public health laboratories, with the capacity to conduct around 10,000 tests per week. In addition, CDC began shipping the tests to some of the largest commercial laboratory companies (including some of the nation’s largest reference laboratories) to increase monkeypox testing capacity. This action improved convenience for patients and healthcare providers across the nation, and the government eased the testing procedures by approving the rapid testing kits for monkeypox. The regulatory bodies approved the products faster and increased access to monkeypox testing, which further accelerated the U.S. monkeypox testing market. In addition, the government took better steps to create awareness, communicate among the LGBTQI+ community, mobilize them, and provide better treatments. It was estimated that the U.S. would require USD 7 billion to handle the monkeypox outbreak.
Request for free sample report: https://www.arizton.com/request-sample/3729
Amid the CDC’s orthopoxvirus test expansion, several diagnostics companies are developing and launching their monkeypox-specific assays. BD, Cepheid, and Roche have joined the race to roll out accurate, PCR-quality tests for the disease. BD has joined forces with CerTest to adapt one of the Spanish company’s existing tests to run on the BD Max automated PCR system. At the same time, Cepheid partnered with reagent maker BioGX to design a molecular assay of its own. Abbott is developing a polymerase chain reaction (PCR) test to detect the presence of monkeypox. The company is focused on developing a monkeypox test, which could help the world in the early detection of the disease. It will provide test kits to partners in the pandemic defense coalition. In addition, F. Hoffman La Roche launches LightMix Modular Virus test kits to analyze samples using quantitative PCR technology. Various companies across the globe are developing monkeypox test kits to increase the testing and to decrease the spread of infection globally.
If our report does not include the information you are searching for, you may contact us to have a report tailored to your specific business needs https://www.arizton.com/customize-report/3729