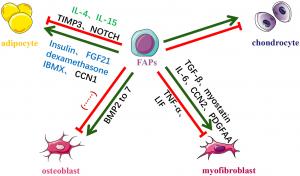
Multi-lineage differentiation of Pdgfrα+ stromal cells in vitro and in vivo. Pdgfrα+ stromal cells have multipotent capacity in different conditions. Representative signals regulating differentiation of Pdgfrα+ stromal cells in vitro and in vivo are liste
SHANNON, CLARE, IRELAND, March 1, 2025 /EINPresswire.com/ -- A recent study has shed new light on the role of fibro-adipogenic progenitors (FAPs) in muscle regeneration, fibrosis, and degeneration. These cells play a crucial part in tissue homeostasis, and their functions have significant implications for conditions such as muscular dystrophy, sarcopenia, and muscle atrophy.
How inflammatory cytokines influence the behavior of FAPs, impacting their proliferation, apoptosis, and differentiation was explored. The role of TNF-α, a key cytokine secreted by Ly6C^high macrophages, which can either promote FAP apoptosis and reduce fibrosis or contribute to muscle degeneration, depending on the state of the muscle was highlighted. Additionally, IL-1α and IL-1β were identified as potent inhibitors of FAP adipogenesis, while growth factors such as betacellulin and epidermal growth factor enhance their proliferation.
One of the most important findings relates to IL-33, a cytokine primarily secreted by FAPs, which plays a crucial role in muscle injury repair by recruiting regulatory T cells. The study also reveals that in denervated muscles, FAPs secrete IL-6 through STAT3 pathway activation, contributing to both muscle atrophy and fibrosis.
The study highlights the potential for targeted therapies to modulate FAP behavior in various muscle disorders. Notably, histone deacetylase inhibitors (HDACi), currently in clinical trials for Duchenne muscular dystrophy (DMD), can induce FAPs to shift towards a pro-myogenic phenotype, improving muscle regeneration in dystrophic environments.
Such research offers new therapeutic directions for managing muscle-wasting diseases and underscores the importance of understanding cellular interactions in muscle repair and degeneration.
Funding Information:
National Natural Science Foundation of China (No. 82173134)
Sichuan Science and Technology Program (China) (No. 2023ZYD0061)
Jinfeng Laboratory (Chongqing, China)
China Postdoctoral Science Foundation (No. 2020M673648)
Chongqing Postdoctoral Science Special Foundation (China)
Chongqing Science and Technology Bureau (China) (No. CSTB2022TIAD-KPX0168)
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3
Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases ).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases )
# # # # # #
Reference
Xia Kang, Kun Zhao, Zhu Huang, So-ichiro Fukada, Xiao-wei Qi, Hongming Miao, Pdgfrα+ stromal cells, a key regulator for tissue homeostasis and dysfunction in distinct organs, Genes & Diseases, Volume 12, Issue 2, 2025, 101264, https://doi.org/10.1016/j.gendis.2024.101264
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.