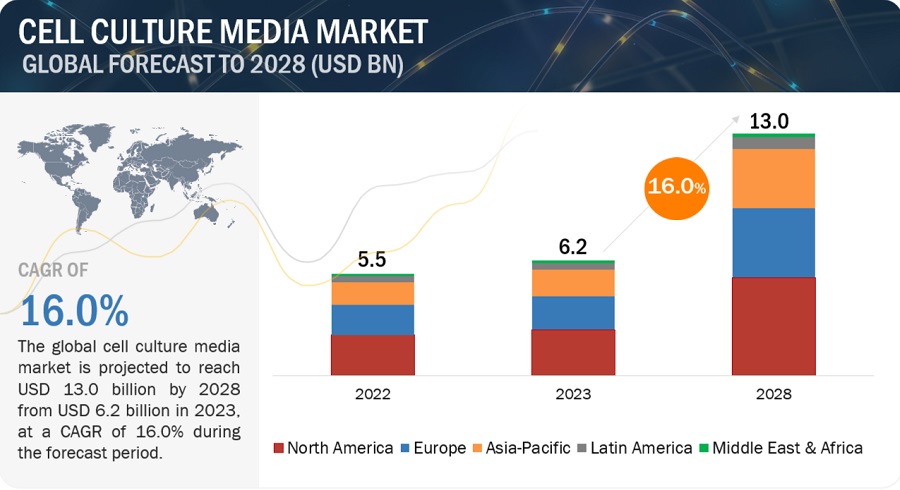
The Global Cell Culture Media Market is projected to USD 10.3 billion by 2026 from USD 4.9 billion in 2021, at a CAGR of 16.0 % between 2021 and 2026. The growth of this market is majorly driven by the rising R&D spending in pharmaceutical companies, emerging cell culture technologies for cell-based vaccines, increasing demand for monoclonal antibodies, growth in stem cell research, the launch of new cell culture media by market players, and the growing focus on personalized medicine. On the other hand, expensive cell biology research products and ethical concerns regarding cell biology research are expected to hinder the growth of this market.
Get Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=97468536
Key Market Players:
Thermo Fisher Scientific, Inc. (US), Merck KGaA (Germany), Danaher Corporation (US), Sartorius AG (Germany), Corning Incorporated (US), FUJIFILM Irvine Scientific, Inc. (Japan), Lonza Group AG (Switzerland), Becton, Dickinson and Company (US), Miltenyi Biotec (Germany), HiMedia Laboratories Pvt. Ltd. (India), STEMCELL Technologies Inc. (Canada), Biologos LLC (US), Cell Applications, Inc. (US)
Global Serum-free Cell Culture Media Industry Dynamics
DRIVER: Emerging cell culture technologies for cell-based vaccines
In the pharmaceutical industry, cell culture has become a prominent part of vaccine production. The cell culture technology has been used to produce vaccines for rotavirus, polio, smallpox, hepatitis, rubella, and chickenpox. Cell-based flu vaccines have also been approved for use in the US and many European countries.
Earlier, vaccines were manufactured by growing and harvesting viruses in chicken eggs, which was a time-consuming process. On the other hand, cell culture-based vaccine production is a far more efficient method. Its advantages over traditional manufacturing methods include a shortened lead-time and the capability to produce vaccines in larger quantities as per demand. Moreover, cell culture-based vaccines can be stored over a longer period. According to a study published by the CDC in the Journal of Infectious Diseases (June 2020), cell-based vaccines provided greater protection against flu-related hospitalizations than standard-dose egg-based vaccines among Medicare beneficiaries 65 years and older. For the 2020-2021 flu season, all four flu viruses used in Flucelvax Quadrivalent were cell-derived. Consequently, recent years have seen a surge in cell-based vaccines, resulting in efforts by prominent companies to capitalize on the opportunities provided. Seqirus, for example, has been active in this field.
- In February 2020, Seqirus (a part of CSL Limited) received US FDA approval for AUDENZ, a cell-based Influenza A (H5N1) Monovalent Vaccine for individuals aged six months and older.
- In January 2019, Seqirus received approval from the European Commission for its new cell-based seasonal influenza vaccine, FLUCELVAX TETRA.
- In October 2018, Seqirus secured FDA approval for a next-generation cell-based influenza vaccine manufacturing process. This approval has enabled the inclusion of children as young as 6 months. Also, after the FDA approval for its cell-based manufacturing technology, Seqirus went from producing 3 million doses in 2015 to 20 million doses in 2020.
Since there is a surge in demand for cell culture-based vaccines, new techniques for vaccine development are emerging. This has subsequently boosted the demand for cell culture media.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=97468536
RESTRAINT: Ethical concerns regarding cell biology research
Research in cell biology necessitates the use of animals and humans, as animal and human cells are used in gene therapy studies that involve the recombination of genes and stem cell research therapies. These cells are also used for in vivo toxicity and pharmacokinetic testing of drugs, which may harm animals and humans. In addition, stem cell research studies use human embryos for clinical applications that often lead to the destruction of human embryos. In order to monitor these activities, strict regulations have been formulated by ethical authorities in various countries across the globe. Authorities such as the Human Tissue Authority (HTA), Human Fertilization and Embryology Authority (HFEA), Medicines and Healthcare products Regulatory Agency (MHRA), and Central Ethics Committee for Stem Cell Research (ZES), among others, have implemented stringent regulations for cell biology research. These ethical concerns and restrictions on the use of cells for research are limiting cell biology research to a great extent in various countries across the globe. This, in turn, is expected to restrict the demand for cell culture products, including cell culture media
North America was the largest regional market for Cell Culture Media Market in 2020.
The global cell culture media market has been segmented into North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. In 2020, North America accounted for the largest share of the cell culture media market, followed by Europe. The growing regulatory approvals for cell culture-based vaccines, growth in the biotechnology & pharmaceutical industries, higher investments in cell-based research, and strong government support are the key factors driving the growth of the cell culture media market in North America.
Recent Developments
- In October 2021, Thermo Fisher Scientific Inc. (US) launched the Gibco Cell Therapy Systems (CTS) NK-Xpander Medium, which supports the large-scale growth and culture of functional natural killer (NK) cells with or without the use of feeder cells.
- In July 2021, Sartorius AG acquired cell culture specialist Xell AG thus expanding its capabilities for manufacturing dry powder and liquid media.
Buy Now: https://www.marketsandmarkets.com/Purchase/purchase_reportNew.asp?id=97468536
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies' revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model - GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets's flagship competitive intelligence and market research platform, "Knowledgestore" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: 1-888-600-6441
Related Reports:
Stem Cell Manufacturing Market: https://www.marketsandmarkets.com/Market-Reports/stem-cell-manufacturing-market-70743403.html
Cell Expansion Market: https://www.marketsandmarkets.com/Market-Reports/cell-expansion-market-194978883.html
Stem Cell Therapy Market: https://www.marketsandmarkets.com/Market-Reports/stem-cell-technologies-and-global-market-48.html
3D Cell Culture Market: https://www.marketsandmarkets.com/Market-Reports/3d-cell-culture-market-191072847.html
Cell Culture Market: https://www.marketsandmarkets.com/Market-Reports/cell-culture-market-media-sera-reagents-559.html