The Aveni Foundation mission is to expedite development of gene-targeted technologies for cancer and COVID-19, an unmet medical need.
PREVENTING RECURRENCE OF TRIPLE NEGATIVE BREAST CANCER WITH TUMOR-TARGETED GENE THERAPY
?This is the second FDA use authorization for DeltaRex-G in preventing recurrence of breast cancer. DeltaRex-G is a viable treatment for patients who opt not to receive standard toxic chemotherapy.”
LOS ANGELES, CALIFORNIA, UNITED STATES, June 27, 2021 /EINPresswire.com/ -- The Aveni Foundation (www.avenifoundation.org), and the Cancer Center of Southern California (www.cancercentersocal.com) are proud to announce that the USFDA has granted authorization for DeltaRex-G as first-line single agent therapy for a patient with early stage triple negative metaplastic carcinoma of breast. Triple negative breast cancer occurs in only 10-15% of patients with invasive ductal carcinoma of breast and is associated with a poorer prognosis than receptor positive breast cancer.— Erlinda M. Gordon, M.D.
In a Phase 1/2 study using DeltaRex-G for chemotherapy-resistant Stage 4 breast cancer, DeltaRex-G induced 17.6% response rate by PET/Choi criteria, 76% tumor control rate and 83% one-year survival rate, with minimal, if any, systemic toxicity, and with 2 patients still alive >10 years after DeltaRex-G treatment initiation (Bruckner et al., Molecular Therapy Vol 27 No 4S1, abstract #273). These data suggest that (1) DeltaRex-G is uniquely safe and exhibits antitumor vshould be used to evaluate efficacy, (3) DeltaRex-G induced long term (>10 years) survival in 2 patients with pure bone metastases who subsequently received DeltaVax immunotherapy, (4) DeltaRex-G may prove to be a biochemical and/or antigen modulator when combined with other cancer therapy/ immunotherapy, and finally (5) DeltaRex-G is a viable alternative treatment for patients who choose not to receive standard toxic chemotherapy that may predispose them to secondary malignancies, debilitating peripheral neuropathy and other untoward side effects that impair quality of life while undergoing treatment for breast cancer.
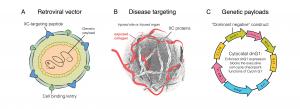
DeltaRex-G is a tumor-targeted gene therapy that (a) displays a Signature (SIG)-binding peptide on its surface for targeting the tumor microenvironment, and (b) encodes a designer killer gene for eradicating cancer cells, starving the tumor from its blood supply and destroying cells that make the cancer cell?s nest (stroma). When injected intravenously, the DeltaRex-G nanoparticles seek out and accumulate in the tumor microenvironment where Signature (SIG) proteins are abnormally found, in the vicinity of cancer cells, hence augmenting effective drug concentration.
From our experience in 5 Phase 1 and 2 US-based and 2 Philippine-based clinical trials for metastatic chemotherapy resistant cancers, we have shown that intravenous DeltaRex-G has minimal, if any, systemic toxicity, and may be effective in prolonging life of Stage 4 cancer patients. According to Dr. Erlinda Gordon, President of Aveni Foundation, ?This is the second FDA use authorization for DeltaRex-G in eradicating microscopic disease and preventing recurrence of breast cancer.? If successful, this FDA use authorization of DeltaRex-G as first line therapy for early-stage triple negative breast cancer establishes a precedent for the use of DeltaRex-G in patients who refuse to receive toxic chemotherapy and radiation therapy. A Phase 2/3 clinical trial is planned to evaluate if DeltaRex-G is equally effective (not-inferior-to) than standard chemotherapy/radiation therapy as first-line adjuvant therapy for early-stage triple negative carcinoma of breast.
To donate for the Breast Cancer clinical trials:
- Click on the DONATE button at www.avenifoundation.org
- Send a check to: Aveni Foundation, 2811 Wilshire Blvd., Suite 414, Santa Monica CA 90403
- Wire funds to: Chase Bank, Account Name: Aveni Foundation, Routing No: 322 271 627 Checking Acct. No.: 317 312 673, SWIFT CHASUS33
For further information, please visit our websites: www.avenifoundation.org, contact Dr. Gordon at egordon@avenifoundation.org or egordon@sarcomaoncology.com. Erlinda M Gordon
Aveni Foundation+1 818-726-3278
Erlinda Gordon
Aveni Foundation
+1 8187263278
egordon@avenifoundation.org
Visit us on social media:
Facebook
Twitter
LinkedIn